Introduction
In addition to the nationally funded COMET – K-Project CCFLOW (2017) the Austrian Research Promotion Agency (FFG) has awarded a two year grant (0.86 Mio €, 2019-2020) termed „SynthesisControl“ to the Research Center Pharmaceutical Engineering GmbH (RCPE). Project partners in this initiative in addition to RCPE are the Institute of Chemistry at University of Graz (Prof. Kappe), the Institute of Automation and Control at Graz University of Technology (Prof. Horn), evon GmbH and Microinnova Engineering GmbH.
Research Objectives
Striving for more sustainable and cost efficient manufacturing processes, the pharmaceutical industry is currently starting to undergo major changes in the way active pharmaceutical ingredients (APIs) are produced. Traditional batch reactors, still predominantly used in the synthesis of organic compounds on large scale, have many important drawbacks. In many instances the safety of the process, product quality or reaction efficiency are compromised. A paradigm shift toward continuous flow processing is seen as a solution to overcome many of these problems. One important benefit of a continuous flow regime is the ease of implementation of process analytical technology (PAT) tools for real time monitoring of critical process parameters and quality attributes. Based on these data, in current API manufacturing streams products not meeting specification are typically diverted to waste. This strategy still increases the pollution associated with the process and ultimately the production costs, but does not yet enhance process efficiency.
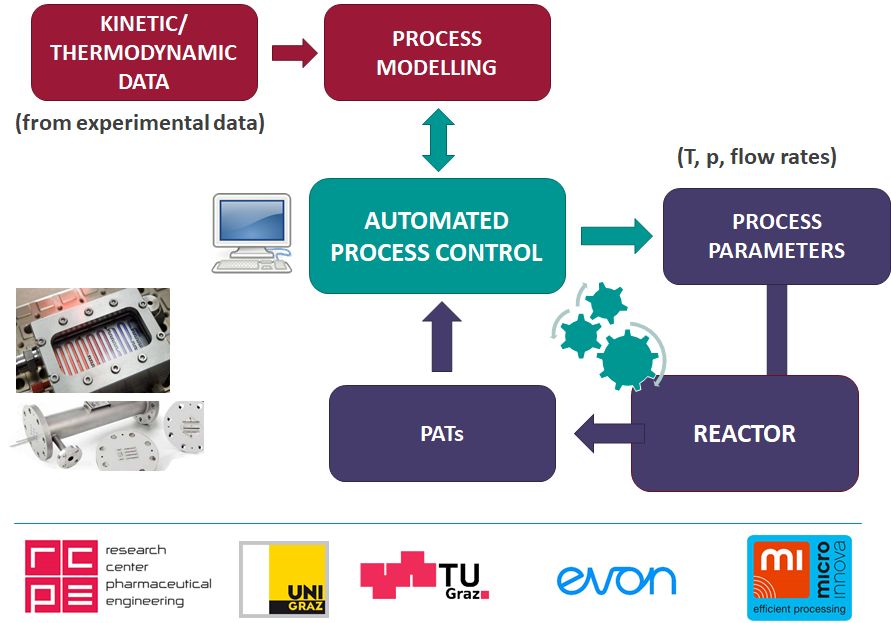
It is the goal of this project to realize a control concept for API synthesis, which is not restricted to diversion of out of spec material. Automatic tuning of the critical process parameters during the chemical reaction, enabled by the control system, will enhance the robustness of the process. An automated control strategy will anticipate system failures or deviations from the quality attributes and react before they occur. Therefore, predictive models will be developed based on fundamental process understanding to design model predictive control strategies. Useful process information extracted from process data continuously obtained from the PAT monitoring of the reaction will be fed back to the controllers. Additionally, the reaction models will further improve during the manufacturing of the API as a “self-learning” type system. The control concept will be implemented and tested in a two stage model reaction processing a widespread API at laboratory scale. All approaches used in the project are universally applicable for different scales or substances. It is expected that translation of the control strategies developed in this project to pilot or large scale production will help to reduce costs and waste generation in the production of pharmaceuticals as well as increase flexibility and robustness of product quality.
Flow Chemistry/PAT Platform
The flow chemistry/PAT systems used for this project will be largely based on the INFRA FLOW platform available at University of Graz. Key component of this infrastructure is a high-performance modular flow chemistry platform from Ehrfeld Mikrotechnik (including MMRS, Lonza FlowPlate and Miprowa reactors) that allows full process automation and intensified flow processing under a wide range of different reaction conditions. This platform is directly linked to various in-line (IR, NMR) and at-line (UHPLC) reaction monitoring tools, or can be used along other flow chemistry equipment available within CC FLOW in order to maximize synergistic effects.
Publications:
Advanced Real-Time Process Analytics for Multistep Synthesis in Continuous Flow
P. Sagmeister, R. Lebl, I. Castillo, J. Rehrl, J. Kruisz, M. Sipek, M. Horn, S. Sacher, D. Cantillo, J. D. Williams, C. O. Kappe
Angew. Chem. Int. Ed. 2021, 60, 8139-8148. DOI: 10.1002/anie.202016007.
Automated and Continuous Synthesis of Drug Substances
S. Sacher, I. Castillo, J. Rehrl, P. Sagmeister, R. Lebl, J. Kruisz, S. Celikovic, M. Sipek, J. D. Williams, D. Kirschneck, C. O. Kappe, M. Horn
Chem. Engin. Res. Design. 2022, 177, 493-501. DOI: 10.1016/j.cherd.2021.10.034.