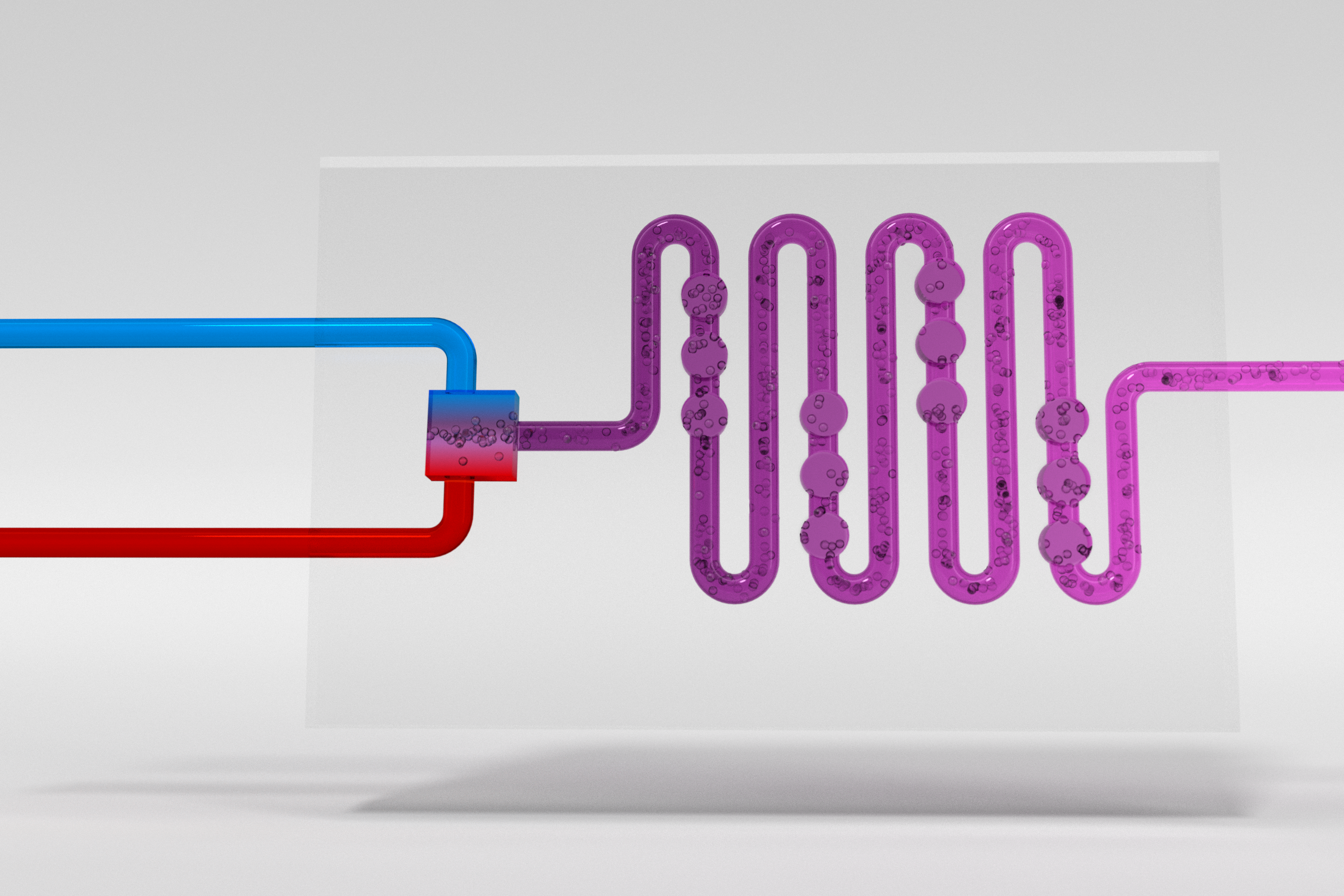
Multiphase Flow Chemistry
Multiphase reactions are very common in the pharmaceutical industry and include, e.g., hydrogenations, oxidations, halogenations and phase-transfer catalysis reactions. They involve the combination of two or more immiscible phases (e.g., liquid-liquid, solid-liquid or gas-liquid reactions). When mass transfer from one phase to the other is the rate-determining step, it is crucial to maximize the interfacial area. In batch, the interfacial area is low and poorly defined, thus leading to prolonged reaction times. Due to the small characteristic dimensions of microreactors, large and well-defined interfacial areas are observed, typically orders of magnitudes higher than in traditional batch environments. These large interfacial areas lead to efficient mass transfer between the two phases. Continuous flow (micro)reactor technology therefore offers the unique possibility to perform multiphase reactions, in particular gas-liquid chemistry, with unparalleled efficiency and process safety. Gases can be dosed into the flow system with precise stoichiometry using mass flow controllers and intense mixing of the gaseous and liquid phase can be achieved. Furthermore, high pressure operation increases the concentration of the gas in the liquid phase. Combustion and explosion hazards are reduced in channels of small diameter and, consequently, reactions can be performed under unusually harsh process conditions in a safe and controllable manner. Our laboratory has extensive experience handling gases in flow, including, but not limited to H2, O3, O2, 1O2, CO, CO2, HN3, CH2N2, BrN3, and H2S.
Key Publications
Review: Membrane Microreactors for the On‐Demand Generation, Separation and Reaction of Gases
C. A. Hone, C. O. Kappe, Chem. Eur. J. 2020, 26, 13108–13117. DOI: 10.1002/chem.202001942
Review: The Use of Molecular Oxygen for Liquid Phase Aerobic Oxidations in Continuous Flow
C. A. Hone, C. O. Kappe, Top. Organometal. Chem. 2019, 377, 2. DOI: 10.1007/s41061-018-0226-z
Review: Heterogeneous Catalytic Hydrogenation Reactions Using Continuous Flow Reactors.
M. Irfan, T. N. Glasnov, C. O. Kappe, ChemSusChem 2011, 4, 300-316. DOI: 10.1002/cssc.201000354
Optimization and Sustainability Assessment of a Continuous Flow Ru-catalyzed Ester Hydrogenation for an Important Precursor of a ß2-Adrenergic Receptor Agonist
M. Prieschl, J. García-Lacuna, R. Munday, K. Leslie, A. O’Kearney-McMullan, C. A. Hone, C. O. Kappe, Green Chem. 2020, 22, 448-454. DOI: 10.1039/D0GC02225J.
Continuous Flow Synthesis of Carbonylated Heterocycles via Pd-Catalyzed Oxidative Carbonylation Using CO and O2 at Elevated Temperature and Pressure
Y. Chen, C. A. Hone, B. Gutmann, C. O. Kappe, Org. Process Res. Dev. 2017, 21, 1080-1087. DOI: 10.1021/acs.oprd.7b00217.
Continuous Flow Difluoromethylation with Chlorodifluoromethane under Biphasic Conditions
B. Gutmann, P. Hanselmann, M. Bersier, D. Roberge, C. O. Kappe, J. Flow. Chem. 2017, 7, 46-51. DOI: 10.1556/1846.2017.00005 .
Batch and Continuous Flow Aerobic Oxidation of 14-Hydroxy Opioids to 1,3-Oxazolidines – A Concise Synthesis of Noroxymorphone (Hot Paper)
B. Gutmann, U. Weigl, D. P. Cox, C. O. Kappe, Chem. Eur. J. 2016, 22, 10393–10398. DOI:10.1002/chem.201601902
A Lab-Scale Membrane Reactor for the Generation of Anhydrous Diazomethane (Featured Article)
D. Dallinger, V. D. Pinho, B. Gutmann, C. O. Kappe, J. Org. Chem. 2016, 81, 5814-5823. DOI:10.1021/acs.joc.6b01190
Shifting Chemical Equilibria in Flow – Efficient Decarbonylation Chemistry Driven by Annular Flow Regimes.
B. Gutmann, P. Elsner, T. Glasnov, D. M. Roberge, C. O. Kappe, Angew. Chem. Int. Ed. 2014, 53, 11557-11561. DOI: 10.1002/anie.201407219
Flash Carboxylation: Fast Lithiation – Carboxylation Sequence at Room Temperature in Continuous Flow.
B. Pieber, T. Glasnov, C. O. Kappe, RSC Adv. 2014, 4, 13430-13433. DOI: 10.1039/c4ra01442a
In Situ Generation of Diimide from Hydrazine and Oxygen – Transfer Hydrogenation of Olefins in Continuous Flow.
B. Pieber, S. T. Martinez, D. Cantillo, C. O. Kappe, Angew. Chem. Int. Ed. 2013, 52, 10241-10244. DOI: 10.1002/anie.201303528
Continuous Flow Synthesis of Adipic Acid from Cyclohexene Using Hydrogen Peroxide in High-Temperature Explosive Regimes.
M. Damm, B. Gutmann, C. O. Kappe, ChemSusChem 2013, 6, 978-982. DOI: 10.1002/cssc.201300197
Direct Aerobic Oxidation of 2-Benzylpyridines in a Gas-Liquid Continuous-Flow Regime Using Propylene Carbonate as Solvent.
B. Pieber, C. O. Kappe, Green Chem. 2013, 15, 320-324. DOI: 10.1039/c2gc36896j
Phase-Transfer Catalysis – Mixing Effects in Continuous-Flow Liquid/Liquid O- and S-Alkylation Processes.
B. Reichart, C. O. Kappe, T. N. Glasnov, Synlett 2013, 24, 2393-2396. DOI: 10.1055/s-0033-1339839
